Abstract
Frailty is a multiply determined, age-related state of increased risk for adverse health outcomes. We review how the degree of frailty conditions the development of late-life diseases and modifies their expression. The risks for frailty range from subcellular damage to social determinants. These risks are often synergistic—circumstances that favor damage also make repair less likely. We explore how age-related damage and decline in repair result in cellular and molecular deficits that scale up to tissue, organ and system levels, where they are jointly expressed as frailty. The degree of frailty can help to explain the distinction between carrying damage and expressing its usual clinical manifestations. Studying people—and animals—who live with frailty, including them in clinical trials and measuring the impact of the degree of frailty are ways to better understand the diseases of old age and to establish best practices for the care of older adults.
Main
In 2014, an influential commentary decried conventional, one-disease-at-a-time approaches to age-related illnesses1. This geroscience view steers away from the ‘whack-a-mole’ approach2 of treating specific diseases individually—a process that often yields further problems including polypharmacy3 and hospital-induced functional decline4,5. Instead, the geroscience agenda envisages moving toward a systems-level approach that slows the aging process2,6. Progress has been made, for example, with human clinical trials of senolytic drugs that target senescent cells7,8. Even so, aging is multiply determined and has many manifestations9,10 meaning that despite any one advance, gaps will remain between the diseases of aging and optimal health in old age11.
These gaps between healthy aging and the diseases of old age reflect important conceptual and operational challenges in continuing to address age-related impairments in health. Major noncommunicable age-related diseases such as cancer, coronary heart disease, dementia, diabetes mellitus and stroke share common risk factors12,13. Their prevention, and improving poor health in old age more generally, requires developing new systemic interventions. Some such systemic interventions already exist, such as tackling the shared and modifiable risks of physical inactivity and poor diet12,14.
Less appreciated has been the opportunity to ‘treat aging’1, which can be operationally framed as an opportunity to ‘treat frailty’11, a proposition that can be indifferent to exactly which aging mechanism is being targeted15. That opportunity arises because detecting widespread effects of existing and future interventions requires integrative measures of age-related health. The degree of frailty, that is, variability in risk, is a widely used, theoretically grounded means of quantifying health in old age. Other candidate integrative measures of health include ‘biological age’ such as provided by ‘physiological dysregulation’16, DNA methylation clocks17,18, telomere length and other aging biomarkers19,20. Frailty is a coarsely grained, system-level measure that can be used across levels from molecular and cellular measures to tissues to organs to whole organisms21—and can also be applied to animals22.
Measuring frailty offers insights into clinical medicine and population health14,23,24. At any age, individuals with high levels of frailty are much more susceptible to adverse outcomes than those with lower frailty scores21. Severe frailty typically occurs more often in women25, is observed in 12–24% of older adults and is age related26. Increasingly, frailty is seen as modifiable, even potentially preventable, thereby making it a target of treatment27,28,29,30,31. Trials of nutrition and physical exercise face challenges in relation to blinding, types of controls and measurement of dosing that can be more challenging than pharmaceutical studies, especially for well-tolerated compounds27. These challenges must be faced in sufficiently large, locally adapted, randomized controlled trials if the prospect of prevention or even mitigation is to be more than aspirational. For this, multicomponent programs will be important, including taking the social context into account29, something not always done31. Even so, caution is required: one indication that the path to success may be quite long is signaled by a multicomponent trial of metformin and exercise—instead of increasing, metformin attenuated some of the benefit associated with exercise32.
In reviewing how frailty relates to disease in old age, we explore how age-related damage and decline in repair in various guises are detectable. We examine frailty and its antecedents from cellular and molecular damage33,34,35,36 to social determinants of health such as education, social position, race and financial stability13,14,23. We consider frailty as a means of understanding variability in aging, in translating interventions from preclinical to clinical studies, and as an aid to clinical decision-making. Finally, we call for the development of new intervention strategies, investigation of frailty mechanisms and use of frailty as an outcome measure in clinical trials and in improving hospital best practices.
Operationalizing frailty in humans and other animals
No one who has attended a thirtieth high school reunion doubts that people age at different rates. In 1979, variability in rates of aging was invoked to explain the apparent decline in the mortality rate at extreme old ages37, positing that eventually only slow agers are left after the frailer rapid agers die. While late-life deceleration of mortality is still debated38,39, the notion of frailty as variability in the risk of death in people of the same age—generalized as variability in the risk of an adverse outcome to people with the same degree of exposure—is an established concept. Describing people as being frail when they appear to be substantially ‘older than their stated age’ is now part of the clinical lexicon40.
In 2001, several papers proposed new operational approaches to frailty41,42,43. Since then, although many frailty tools have been developed, two approaches have predominated (Table 1)44,45,46. One sees frailty as conforming to a phenotype or syndrome that underscores physical decline: low levels of grip strength, slow walking speed, loss of weight, reduction in usual activities and feeling exhausted; any three of these five features define a person as frail41. With the frailty syndrome, the degree of frailty can be expressed as the number of the five attributes that are present, which allows a six-point scale.
The other approach, developed by our group, sees frailty not as a specific syndrome but as a more general age-related state of poor health that is proportional to how many age-related health deficits an individual has accumulated42. Deficits can be drawn from a variety of attributes or functions, the relevance of which can vary depending on the context (for example, deficits related to exposure to malaria, frostbite or prolonged mechanical ventilation). The degree of frailty for any individual can be expressed as the fraction of deficits that are present in an individual to the number that were considered in a standard clinical or epidemiological study. If at least 30 age-related and adverse-outcome-associated items are considered, the degree of frailty, for the most part, does not depend strongly on which items are counted21,47,48.
The operationalization of each approach can vary considerably (Table 1), but the results remain robust. Not all five items in the frailty phenotype are present in every database or clinic record, so that sometimes only four are used49. Sometimes self-reported data or different questions than those originally proposed are used49. The FRAIL scale, a self-report version, to which an item about the number of comorbidities has been added, is a popular variant50. The original frailty phenotype is sometimes referred to as ‘physical frailty’, to distinguish it from similarly constructed frailty syndromes for cognitive frailty51, social frailty52 and organ-specific frailties53. All of these are constructed with specific health deficits that typically increase with age and individually are associated with adverse health outcomes.
The frailty index is also subject to variable operationalization. Although a frailty index can be derived from any combination of symptoms, signs, laboratory values or other measures42, work with a frailty index developed from laboratory data54 or biomarkers55 suggests quantitative differences in the distributions of the data, and slopes and intercepts in relation to age (Table 1). Other variations include the Clinical Frailty Scale, which proposes ordered combinations of high-information deficits to grade the degree of frailty56. The ‘modified frailty index’, especially in the American surgical literature, includes measures, mostly comorbidities, using a smaller number of deficits, such as a modified frailty index with 11 items (Table 1). However, such shorter versions have been criticized as being too brief to constitute a frailty index based on deficit accumulation5,57.
The differences between frailty as a syndrome and frailty as a state of deficit accumulation, although real, are easily exaggerated. Each has been used in a variety of applications, including at the population level58,59,60,61. Both approaches are informative: at the group level, they consistently classify people who are at an increased risk of death and do so in ways that tend to reduce the explanatory power of age62. They appear to share genetic determinants63. Fundamentally, what the two main approaches have in common is that each sees frailty as rooted in aging. Each captures that not all people age at the same rates and that not everyone of the same age has the same risk of death. Rather than searching for a single aging biomarker, each approach uses more than one feature to define frailty. For each, once frailty is characterized, effort can be made to explore the antecedents of differential aging. Some antecedents will be risk factors for differential aging (such as genetic influences or social vulnerability), while others will be features of aging, such as multiply determined loss of the ability to withstand stress (the idea of robustness64) or to remove or repair damage when it arises (the idea of resilience64). The loss of either robustness or resilience is seen as arising from the diminution in ‘physiological reserve’. A common unifying definition of frailty includes both robustness and resilience. Frailty is an age-related, multiply determined loss of ability to respond to common stressors.
Preclinical frailty models have been developed from both the phenotypic and accumulation-of-deficits approaches;22 so far, the best studied are murine models. Some items correspond to integrative attributes (such as gait speed, usual activities41, health attitude, role function, instrumental activities of daily living and mobility in people42,43,45,65; and gait speed, grooming, body temperature, body condition score and menace reflex in mice66,67,68), although more focused attributes are also available (such as hip flexor strength, cataracts, hearing loss and specific comorbidities in people; and hearing loss, kyphosis, cataracts and tumors in mice67; Table 1). Frailty tools have also been developed for use in dogs69,70 and nonhuman primates71. Quantifying frailty with similar approaches in both clinical and preclinical studies will facilitate translational research.
The degree of frailty contextualizes changes during aging
While frailty inspires investigators, this enthusiasm is not universal. Some are quoted as seeing in frailty "a pejorative concept that validates and reinforces the disadvantage and vulnerability of aging adults"72. Frail patients have multiple, interacting, medical and social problems that confound the single-problem treatments for which much of contemporary health care is organized73. They can be characterized by a physician as ‘unsuitable’ for care74. Nevertheless, growing evidence suggests that the degree of frailty helps us to understand not just risk, but the expression of chronic and acquired diseases in older people.
Indeed, a higher degree of frailty has been linked to a greater risk of disability in activities of daily living and falls75,76, delirium77,78,79, as well as more hospital admissions, with longer lengths of stay75,80, and more primary care visits—all leading to greater health care costs73,81. Work on the degree of frailty and risk extends to a variety of clinical settings, including critical care5, during invasive interventions46 and in nursing homes82.
Frailty and COVID-19
Recent experience with coronavirus disease 2019 (COVID-19) illustrates how frailty can integrate risk. Mortality in COVID-19 is related to the degree of frailty83,84,85,86,87, however operationalized88. The mortality data from COVID-19 reflect the known dose–response relationship between the degree of frailty and the risk of death58. Similarly, frailty is related to the risk of COVID-19 being severe89 and to important complications, including incomplete recovery90 and prolonged hospital stay85,86. Frailty can also be more common in people with COVID-19 who develop delirium; mortality is especially high in this setting84,91. Indeed, new onset delirium may be a presenting symptom of COVID-19 (ref. 92). That delirium is associated with frailty93,94 illustrates why patients who live with frailty can be perceived as unsuitable. By not being able to describe what is wrong with them, frail patients who are delirious fail to engage providers at the typical point of encounter in health care95. As a result, delirium is underdiagnosed95. Among the patients with delirium, 37 (16%) had delirium as a primary symptom, and 84 (37%) had no typical COVID-19 symptoms or signs, such as fever or shortness of breath96. Despite the toll taken by COVID-19, the increased risk of adverse outcomes noted above and the potentially negative impact of frailty on the response to vaccination97, frail older adults are underrepresented in vaccine trials98,99.
Frailty and the risk of dementia in Alzheimer disease
Alzheimer disease and late-life dementia illustrate how measuring the degree of frailty can allow a deeper understanding of the relationship between aging and important diseases of old age. By 2011, it was evident that clinical trials designed to prevent the accumulation of the form of the beta-amyloid protein most associated with dementia pathologically were not working. A move was made to distinguish between Alzheimer disease as a biomarker-defined entity, and Alzheimer dementia as a clinical syndrome100. Not everyone with phenotypical Alzheimer disease demonstrated that they carried the toxic form of amyloid101—the resulting ‘lack of target organ engagement’ was understood as the reason for much of the failure rate of anti-amyloid therapy102,103. Drugs could result in amyloid plaques being cleared from the brain, without any detectable impact on cognition103,104. Indeed, pure Alzheimer disease is uncommon; instead, most older adults with dementia have multiple neuropathological markers105,106,107,108. Community-based neuropathological studies show that not everyone who meets clinical criteria for Alzheimer disease has dementia, and not everyone who meets dementia criteria meets the neuropathological criteria109,110. Instead, a host of other features, including age, atrophy and social position come into play.
Age-associated health deficits that do not include known dementia risk factors (for example, stroke, slow motor speed and functional impairment) nevertheless increase the risk of late-life cognitive impairment111 and dementia112. Even people with a high burden of Alzheimer disease pathology are at less risk of meeting criteria for dementia if they have low frailty scores110 (Fig. 1). In late-life dementia, it is now appreciated that neuropathological markers denote risk but do not permit a definitive diagnosis of cognition before death. Risks of all-cause dementia are additive when considering neuropathological markers of dementing illnesses113 and known risk factors act even more potently in the face of frailty114. Risks for frailty and dementia overlap—especially in relation to social position, education and physical activity14,115.
The pathological changes in the brain that are thought to increase dementia risk include plaques, tangles, Lewy bodies and ischemic changes. Individuals with marked Alzheimer disease pathology may not meet criteria for dementia if they are fit, with low levels of frailty, while those with a modest neuropathological burden are at increased risk for dementia if they have a high degree of frailty. This figure was modeled on data in refs. 14,110,112,113,114,157.
Frailty and clinical cardiovascular disease
Clinical studies show that the degree of frailty is related to the risk of various outcomes of cardiovascular disease, such as myocardial infarction, stroke, heart failure or atrial fibrillation 116,117,118,119. For example, using clinical and test data, a 34-item frailty index was constructed from which were excluded deficits that were traditional risk factors for cardiovascular disease. The resulting 26-item frailty index was compared with traditional risk as assayed using the Framingham risk score118. Each 0.1 increment in the frailty index increased the hazard ratios for both cardiovascular and non-cardiovascular mortality. Frailty was associated with a greater risk of both cardiovascular events and mortality, independently of traditional cardiovascular risk factors.
A meta-analysis of older adults with atrial fibrillation revealed that fewer fitter patients living with severe frailty were given oral anticoagulants for stroke prevention than were fitter people with atrial fibrillation120. A study using a records-based electronic frailty index121 confirmed that the risk of atrial fibrillation, death and gastrointestinal bleeding (and among women, stroke) all increased with the degree of frailty122. Prescription of oral anticoagulants increased with the degree of frailty except in those with severe frailty so that fitter people had lower rates of oral anticoagulant prescription than did their frailer peers122. In a post hoc analysis of a clinical trial of a direct oral anticoagulant medication (edoxaban) in patients with atrial fibrillation, each 0.1 increment in the frailty index was associated with a greater risk of stroke or spontaneous embolism, and of major bleeding123. Patients receiving edoxaban had a similar benefit from oral anticoagulation as did those receiving the more traditional drug warfarin, but a lower risk of bleeding, save in people living with severe frailty. Thus, the degree of frailty influences responses to cardiovascular medications, including those frail older individuals who are the most likely to take these drugs.
Frailty and outcomes of hypertension
A similar body of work exists relating the degree of frailty with hypertension. Frailty indices have been calculated both retrospectively124 and prospectively125 in clinical trials of antihypertensive medications. Using frailty to identify and control for heterogeneous populations of older adults125 led to recommendations for aggressive blood pressure control even in frail older adults. Even so, those same guidelines have been criticized on the grounds that they do not generalize to the general population, given how restrictive typical clinical trial enrollment criteria are, even when frailty may have been measured126,127,128. For this reason, guidelines have been proposed to move with great caution in people living with severe frailty or dementia127,128.
The paradox of excluding those most at risk from clinical practice guidelines
In addition to brain and cardiovascular disease, and COVID-19, frailty and disease-specific risk factors overlap in disease progression in a variety of illnesses, including osteoporosis129, HIV/AIDS130 and systemic lupus erythematosus131. Despite this, excluding patients who live with higher degrees of frailty from clinical trials is a common, if derided, practice98,99,132,133,134. Nevertheless, people living with mild to moderate frailty find their way into trials and bring with them a higher risk of adverse outcomes135. In consequence, it has been recommended that people who live with frailty merit closer monitoring135, and that trials in chronic diseases in which frailty is common should determine which treatments frailer patients might tolerate best136. Indeed, in many subgroups, including younger people, patients with higher degrees of frailty appear to be more likely to benefit from treatment.
Context matters, and especially in relation to excluding older adults who live with frailty; informative context also extends to social and economic settings137,138, race13,86,139 the clinical setting5,46,82,86,140, childhood influences141, and cohort and period effects13,142,143,144,145,146.
Frailty and age-related deficit accumulation
Deficit accumulation leading to increased frailty occurs across the human life course. Early life influences are reflected in birth cohort studies147,148 and cross-sectional population studies from ages 20 and younger149,150. Country-of-origin studies151,152,153 also reveal important effects. Those using data on related childhood socioeconomic conditions141,154,155 also highlight the importance of what happens in childhood. Genetic and proteomic studies have been done in special populations, such as in twin studies156,157,158 including adopted twins raised apart19, or in comparing offspring of people who come from long-lived families with those whose parents had usual survival patterns159. Briefly, as summarized in a detailed 2020 review63, the heritability ranges from 25–30% (using phenotypic and deficit accumulation measures)160 to about 45%156.
Together, these studies suggest that genetic, environmental and social effects operate broadly and affect frailty (Fig. 2). Note that we do not have a cohort study in which individuals have been followed from birth to complete mortality, so some inferences from what data we do have are needed.
The degree of fitness or frailty in an individual is profoundly affected by lifestyle factors, social factors and medical interventions. Dietary modifications (for example, calorie restriction), exercise, social engagement, education and drug therapies (for example, geroprotectors, senolytic drugs and repurposed drugs) have been shown to attenuate frailty in preclinical and/or clinical studies. Other lifestyle factors (for example, stressful environment and high-fat diet) and interventions including medical treatments (for example, radiation therapy and polypharmacy) can make frailty worse. Factors such as low social position, limited personal wealth, poor maternal health, low levels of childhood education, low per-capita gross domestic product and a host of specific disease states increase the prevalence and consequences of frailty in affected populations.
Note too that, just as with the genetics, no single influence amounts to destiny. For example, although immigrating from a lower-middle-income country to a high-income Northern European country showed a deficit never entirely caught up, people moving to Southern/Eastern Europe and people from lower- and middle-income countries were no worse off151. Somewhat more optimistically, and also from the Survey of Health, Ageing and Retirement in Europe, the impact of childhood socioeconomic conditions on frailty at old age could be mitigated by better conditions in adulthood: improving socioeconomic conditions can reduce health inequalities in old age155. Unsurprisingly, many influences will vary across the life course148,151,155,158.
At the individual level, these influences give rise to deficit accumulation through a variety of mechanisms, including intrinsic processes that result in damage going unremoved or unrepaired15,19,161,162. Such damage is detectable across the levels at which maladaptive aging-related changes can be observed, for example, with decline in telomere length163, mitochondrial DNA abundance164 or DNA methylation changes18,19,162. Deficits arising from disruptions in cellular and molecular processes then affect tissues, promote organ dysfunction and lead to clinical manifestations and frailty21,162. Evidence that the accumulation of subcellular deficits heralds the development of clinical frailty includes studies with a frailty index (FI-Lab) created from routine laboratory tests and blood tests54,150,165 or from biomarkers55. Higher FI-Lab scores are also seen in people who live in more stressed circumstances166.
Deficits accumulate at a constant rate, doubling roughly every 12–15 years167,168. The resulting pattern of accumulation shows acceleration in the number of deficits in later life, suggesting that deficits do not accumulate independently: people who enter old age with fewer deficits will accumulate fewer, and those who enter with many deficits will accumulate more, as longitudinal studies show169. In consequence, even small differences in early life can have increasingly larger impacts across the life course, even into late old age. Further, within-person acceleration in the frailty index score can appear as a preterminal event170. Factors that affect deficit accumulation include sex/gender, education, maternal health, social position, race, financial stability, childhoodfrailty states, early signs of chronic inflammation and a host of specific disease states, together with the complex relationships between factors13,86,137,138,139,148,170,171. Different studies report differing mean frailty scores. Secular effects on the lethality of frailty appear to be important. Most studies13,143,144 but not all146 report decreasing lethality in relation to frailty. In general, even with secular improvements in the mean degree of frailty13, disadvantaged groups do less well than advantaged ones. Disadvantage is related to race, as well as to social disparities13,41,139,151,152,153,166. Some of the greater lethality of frailty in disadvantaged groups can be linked to a higher pathogen burden (for example, cytomegalovirus, human immunodeficiency virus and human papilloma virus) through a variety of mechanisms that consist of either greater exposure or less ability to mitigate the burden172.
Frailty from molecular to organismal scales
Heterogeneity in effects of aging is detectable in cellular and molecular processes
The degree of frailty affects the risk of adverse outcomes and treatment responses in a variety of cardiovascular diseases. Consistent with the geroscience hypothesis, frailty may set the stage for such diseases before they present themselves clinically. For these inquiries, animal models are well suited (see below), given that frailty increases with age and is associated with adverse events including increased mortality in mice, rats and dogs66,67,69,70,173,174,175,176,177.
In the cardiovascular system, links between chronological age, frailty and maladaptive changes in heart function have been investigated in mice of different ages. On average, the ability of the heart to contract deteriorates with age35,36. Unsurprisingly, there is also considerable interindividual variability—not all older mice exhibit age-associated deterioration in function35,36. When measures of cardiac contraction are plotted as function of an animal’s frailty index score rather than age, a linear relationship is revealed where the frailest mice exhibit the most profound dysfunction35,36. This may underlie why frail older individuals develop exercise intolerance and heart failure, as seen clinically178. Similarly, the speed with which electrical impulses are conducted across the atria declines with age, an effect clearly evident in mice with high levels of frailty33,34. Slowed atrial conduction then provides a substrate for the development of arrhythmias, like atrial fibrillation, that are common in frail people179. We summarize the heterogeneity of age-associated changes in function and how these changes are graded by frailty scores in Fig. 3.
Schematic illustrating the marked heterogeneity in the effects of age on structural and functional parameters (left). This illustrates that age-associated, detrimental changes in function reflect average responses, but many older individuals have function equal to or better than younger adults. When these parameters are plotted as a function of frailty index scores rather than chronological age, responses are closely graded by frailty (right). This figure was modeled on data from our previously published work33,34,35,36.
The effects of frailty are seen across physiological scales
Poor overall health, quantified with a frailty index, predicts functional decline at the organ level in experimental models. These models provide the opportunity to explore how deficits might arise at the cellular and subcellular levels, then scale up to adversely affect function at the organ and system levels. This idea has been tested most fully in the heart. The heart contracts less forcefully in frail mice because there is less calcium influx to trigger contraction in individual heart cells; this is attributable to fewer calcium channel proteins in the heart cell membrane35. There are also posttranslational modifications in the contractile proteins themselves that are graded by the level of frailty in older mice36. Slower electrical impulses in the atria arise from connective tissue deposition, known as fibrosis, and this in turn arises from increased collagen secondary to changes in enzymes involved in extracellular matrix remodeling33,34. These cellular and subcellular modifications are also graded by the degree of frailty. Clinical studies where the degree of frailty is quantified as the frailty phenotype have similarly shown that advanced glycation end products (AGEs) that arise in chronic kidney disease bind to receptors in skeletal muscle180. This leads to capillary rarefaction that may contribute to sarcopenia and physical frailty180. The idea of scaling by the degree of frailty, where deficits arise at the microscopic level and then scale up to the macroscopic level to affect function at the organ and organism levels, is illustrated in Fig. 4.
Left, accumulation of subcellular damage such as collagen deposition in the atria and reduced calcium channel expression in the ventricles results in atrial fibrosis and impaired cardiac contraction, respectively. These changes may promote chronic diseases like atrial fibrillation and congestive heart failure, which can ultimately lead to system failure and frailty. Preclinical studies show that age-related changes in the heart are closely graded by the degree of frailty, so that for both young adult mice (7–12 months, depending on the study) and aged mice (>22 months), those with high frailty scores have the most subcellular damage. Right, the accumulation of AGEs in aging can reduce blood supply in skeletal muscle, leading to sarcopenia and impaired physical performance. Clinical studies indicate that individuals with high levels of frailty exhibit more AGE accumulation and functional impairment than those with lower frailty scores. This figure was modeled on data in refs. 33,34,35,180.
Translational potential of frailty
Animal models of frailty
The ability to quantify the degree of frailty in humans and other animals can facilitate translational geroscience research. Although frailty assessment tools are used to investigate the association between frailty and age in different species, few studies have explored animal frailty across the life course. Estimates now suggest that frailty index scores increase in a similar fashion from early to late life in mice and in humans, and there is a strong link between frailty and mortality at all ages67,174.
Although women generally live longer than men, they are frailer at most ages, a phenomenon called the ‘sex–frailty paradox’25. Studies in older mice and dogs also report that females have higher frailty scores than males69,181,182,183. While clinical studies suggest that behavioral and social factors are also involved in this sex–frailty paradox25, work in preclinical models could help identify biological mechanisms.
Quantifying the degree of frailty can be used to explore fundamental mechanisms involved in its development. For example, neutrophil dysfunction increases with age but is highest in frail older people184. There is also emerging evidence that older mice with high levels of inflammation are frailer than mice of the same age with low inflammation183,185. Future work should investigate inflammation and other hallmarks of aging9,10 to address fundamental questions about how frailty accumulates.
Preclinical models of frailty offer advantages. When compared with their equivalent human scales, both the frailty phenotype and frailty index tools exhibit similarities in the items of which they are composed (Table 1). These measures are responsive to frailty interventions and are relatively easy to administer. Further, these tests are noninvasive so they can be used in longitudinal studies to track the impact of interventions over time for a given individual. Nevertheless, existing preclinical models have limitations. For example, lifestyle, environmental and social factors that profoundly influence the degree of frailty in humans have yet to be investigated in preclinical models. In addition, deficits in domains such as cognition and activities of daily living have not yet been included in preclinical tools (Table 1). Future studies should further refine these instruments to better model the breadth of human frailty during aging.
Testing frailty interventions in preclinical models
Frailty assessment in preclinical models provides a translational platform to test new frailty interventions (Table 2). This can include interventions that attenuate frailty as well as those that exacerbate it. It is well established that voluntary aerobic exercise and high-intensity interval training reduce frailty in animal models (for example, in refs. 186,187,188,189). Known longevity interventions, including calorie restriction, antioxidants (for example, resveratrol) and mechanistic target of rapamycin (mTOR) inhibitors (for example, rapamycin) also reduce frailty in naturally aging mice181,190, as well as in genetically manipulated mice191,192 and nonhuman primates71. In contrast to these findings, a ketogenic diet that mimics calorie restriction has little effect on the level of frailty in aging mice193. Other dietary interventions like protein restriction194 or intermittent fasting195 also reduce frailty or components of frailty, but only in male mice. This indicates that the effects of frailty interventions can be sex specific, which is important as many intervention studies have used only one sex—generally male (Table 2).
Other approaches to mitigate the degree of frailty have been investigated. Hematopoietic stem cell transplantation using new transplantation technology can both increase life span and reduce the degree of frailty196. Drugs that inhibit the renin–angiotensin system (for example, the angiotensin-converting enzyme inhibitor, enalapril) reduce FI scores in aging mice182. Enalapril also reduces biological age and increases life expectancy, as shown by two new ‘clocks’ estimated from FI scores using machine-learning techniques197. Known longevity interventions (for example, methionine-restricted diet) also reduce frailty, lower biological age and increase life expectancy197. Dietary supplements like alpha-ketoglutarate or allicin (a compound derived from garlic that inhibits inflammation) attenuate frailty in aging mice198,199. This suggests that currently approved drugs and supplements may be repurposed to treat frailty in people. There is still much work to be done here as most drugs identified as geroprotectors for use in clinical studies27 have not yet been investigated for effects on frailty. Preclinical models of frailty can also be easily used to investigate whether combination therapies (for example, drug treatment plus an exercise regimen) may better attenuate or even reverse frailty accumulation in aging.
While some interventions can attenuate frailty in preclinical models, others can make frailty worse. For example, genetic disruption of mTORC2 in the brain impairs glucose homeostasis and increases frailty200. In addition, when aging mice were treated with five commonly used medications to model polypharmacy, their frailty scores increased201. Interestingly, de-prescribing reversed this effect on frailty, which suggests that de-prescribing may be a viable strategy to combat frailty in older adults201. Other work has shown that sublethal whole-body irradiation causes premature frailty, which has implications for the long-term health of patients with cancer202. Mice fed a high-fat diet showed an increase in body weight in both sexes, but frailty increased only in male animals181. This sex difference could reflect male–female differences in the ability to resist a stressor, although additional work to generalize this result is needed.
Computational models can facilitate translational research
Aging individuals are complex, interconnected systems. This interconnectedness implies that predicting the specific effects of multiple interventions (for example, deleterious polypharmacy, but also useful interventions) in the face of multiple ailments (multimorbidity) for a particular aging individual will be impossible without embracing the system complexity. Computational models that embrace the complexity of aging (Box 1) will help us to translate results from animal and cellular models to human medicine, and to improve the life course of individual adults at any given point in their life by appropriately choosing from a suite of treatment options. Dynamical models that include interactions between cellular, laboratory and clinical scales of function over individual lifetimes will help us to understand the benefits of specific therapies. Personalized medicine for aging individuals—one that respects individual priorities and capacity for important lifestyle change—will also be facilitated by a deeper understanding of the complexity of aging. Optimized treatment choices and timing to best extend or improve the health span of individuals may then be possible.
Conclusion
As we age, each of us moves closer to death—although not everyone of the same chronological age has the same risk of death. Heterogeneity in rates of aging motivated the idea of frailty. The complexity that underlies heterogeneity in aging reflects its multiply determined nature. This complexity is belied by striking regularities: everyone accumulates health-related deficits with age; women live longer on average than men do, although often in worse health; poor people tend not to live as long as those who are very well off; and everyone dies. Understanding frailty is motivated by two goals: to finely grade risk, and to understand the basis of differential risk, both with a view to modifying or managing it. How should we proceed?
Frailty helps us manage risk
As a clinical construct, frailty identifies people whose age-related health status puts them at greater risk than their aging peers. That risk can be graded by the degree to which someone is frail. The degree of frailty can be practically operationalized both for only a few key variables (as in the frailty phenotype or the Clinical Frailty Scale) and with many variables (as in the frailty index). Greater frailty correlates with worse outcomes58,84,87,118,203,204. The degree of frailty can thereby inform clinical decision-making, where prognosis is the key. This is especially true regarding informed consent about procedural risk or tolerability of chemotherapeutic regimens. Better informed consent follows from better risk gradation for specific interventions. For example, if an intervention transiently increased the degree of frailty by about 0.3, then the chance of dying can be better quantified with respect to the baseline frailty because mortality is high when a frailty index score approaches 0.7 (ref. 205).
Knowing that increased frailty increases risk does not mean that the increased risk is irreversible. The elements that are giving rise to frailty for that individual can be treated or managed. Especially for elective interventions, undertaking pre-procedural management and prehabilitation may offer risk mitigation206,207. Monitoring outcomes by the degree of pre-intervention frailty and the risk of the procedure could also incentivize innovations in care. Some of that innovation might simply be knowing which days after an intervention are the riskiest, with attention being paid to the types of adverse events that arise in relation to the degree of frailty. As attention is increasingly paid to frailty treatment208, there will then be a need to understand what represents a clinically meaningful treatment effect209,210,211.
This approach to quantified risk can be extended to hospital care. Any number of routine hospital practices are often accepted even though they exacerbate risk—for example, being malnourished, lonely, in pain, unnecessarily immobilized, deprived of sleep, over-sedated, otherwise over-medicated, not having personal agency or being exposed to intermittent fear-inducing events. Multicomponent interventions that target such features31, such as the Hospital Elder Life Program, are effective in improving outcomes212, including delirium, for example, by applying cognitive screening, physical and social measures for delirium prevention, and reducing medications likely to increase delirium risk213. Indeed, understanding the relationship between frailty and delirium, and frailty and dementia, offers a pragmatic means to reduce dementia incidence214,215. A nuanced understanding is needed to strike a judicious balance between therapeutic adventurism and nihilism.
Frailty provides context for age-related changes
Measuring the degree of frailty helps us understanding the extent to which mechanisms of aging operate. We can understand how mechanisms change with age. We can also understand how some changes have more widespread effects than others216. This synergizes with the geroscience agenda of treating aging mechanisms that have wide-ranging effects, following the example of exercise or diet.
The geroscience theory of fundamental aging processes posits that only a few key processes underpin how age-associated diseases arise: chronic ‘sterile’ immune activation; macromolecular dysfunction (from DNA damage to protein misfolding and mitochondrial dysfunction); stem, progenitor and immune dysfunction; and cellular senescence10. The theory posits that treating any one mechanism should affect the rest as well216. However, to avoid diluting the impact of separate interventions, some way must be found to integrate different treatment effects. The wide range of potential individual effects from treating fundamental aging processes obliges multicomponent measures so that the benefits, not just the problems, of old age can come as a package1. Frailty provides such a broad multicomponent measure.
Of course, many other quantifiable approaches to summarizing the effects of aging exist. They will offer complementary information to understanding the degree of frailty or the overall biological age, based on tailored features that explicitly relate to putative aging mechanisms. Moving forward, cohort studies can provide that level of detail for new aging treatments and relate them to clinically detectable grades of frailty63,217,218,219. A focus on multimorbidity to study the treatment of what are termed ‘aging-related rather than disease-specific outcomes’ is already under way220, as is a testing program of established interventions from the US National Institute of Aging in a genetically heterogenous mouse model220,221,222.
The complexity of aging can be addressed in large-scale animal studies of naturally aging animals. Mouse models currently predominate, given their suitability for genetic manipulation. Diversification into studies of other animals, especially rats, nonhuman primates and companion animals, would help to translate interventions and clarify our understanding of how human aging is both similar to and distinct from animal models of aging research.
Quantitative models of aging can also advance our understanding. Frailty invites consideration of how measurable aspects of health interact. Approaches and techniques borrowed from other disciplines, such as complex networks, information theory, queuing theory and machine learning, can be used to understand how the degree of frailty is related to change in frailty states or mortality. While a specific health deficit can be understood as a cumulative imbalance between damage and repair, measuring these processes directly over individual life courses is not simple. Quantitative models will let us separately consider sources of damage, including the social environment, from factors that facilitate resisting damage such as vaccination, from factors that facilitate repair such as health care.
The impact of aging on health
Policymakers and the scientific community have been exhorted to prepare for an aging population. Often, attention is drawn to some disease becoming more common as the population ages, so that progress relies on studying how that illness arises, and how to treat it. Seldom do we consider how diseases become more likely to manifest as an individual ages, beyond a cumulative exposure to risk. Even so, we must conceptualize, measure and mitigate the impact that aging has on health. The geroscience agenda has advanced the conceptualization and is aimed at developing treatments. Frailty provides a way of measuring the impact of aging on health. It embraces the complexity of aging in ways that can make its heterogeneity comprehensible. The measure of successful interventions to mitigate the impact of aging on health will be to reduce frailty at the individual and population levels.
Here we have shown that quantifying the degree of frailty addresses two issues raised as fundamental—one in geriatric medicine, and the other in geroscience. The geroscience agenda rests on the assertion that with aging comes myriad changes that impact on any one disease of aging. In basic science investigations of the heart, we see that many of the changes attributed to aging not only arise in old age, but also can be seen in middle age, where they influence disease expression, a feature seen in humans too. Understanding the degree of frailty can add value in considering the heterogeneity of changes in cardiovascular, cognitive and sensory function, even in the presence of other measures of biological age. The degree of frailty also appears to be responsive to a variety of interventions, including in preclinical models. In geriatric medicine, the complexity of aging is reflected in measures that can usefully summarize information to quantify the degree of frailty. That information in turn can be used to target interventions and, using the information gathered in understanding baseline frailty, to develop individualized care plans that embrace the complexity of frailty.
References
- 1.
Fontana, L., Kennedy, B. K., Longo, V. D., Seals, D. & Melov, S. Medical research: treat ageing. Nature 511, 405–407 (2014). This influential commentary pithily summarized the geroscience agenda: "the problems of old age come as a package".
- 2.
Epel, E. S. The geroscience agenda: toxic stress, hormetic stress and the rate of aging. Ageing Res. Rev. 28, 101167 (2020). This paper calls to attention the role of hormetic stress in rates of aging.
- 3.
Davies, L. E. et al. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J. Am. Med. Dir. Assoc. 21, 181–187 (2020).
- 4.
Eckart, A. et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open 9, e026923 (2019).
- 5.
De Biasio, J. C. et al. Frailty in critical care medicine: a review. Anesth. Analg. 130, 1462–1473 (2020).
- 6.
Campisi, J. et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019).
- 7.
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019). This paper demonstrates that selectively ablating senescent cells with senolytic drugs can improve physical dysfunction in aging.
- 8.
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
- 9.
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013). This paper proposed that aging could be defined by characteristic features termed hallmarks.
- 10.
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014). This paper proposed that aging could be defined by characteristic features termed pillars.
- 11.
Howlett, S. E. & Rockwood, K. Ageing: develop models of frailty. Nature 512, 253 (2014).
- 12.
Peters, R. et al. Common risk factors for major noncommunicable disease, a systematic overview of reviews and commentary: the implied potential for targeted risk reduction. Ther. Adv. Chronic Dis. 10, 2040622319880392 (2019).
- 13.
Abeliansky, A. L., Erel, D. & Strulik, H. Aging in the USA: similarities and disparities across time and space. Sci. Rep. 10, 14309 (2020). This paper demonstrates the use of the frailty index as a biological foundation of health economic theory.
- 14.
Hoogendijk, E. O. et al. Frailty: implications for clinical practice and public health. Lancet 394, 1365–1375 (2019).
- 15.
Rutenberg, A. D., Mitnitski, A. B., Farrell, S. G. & Rockwood, K. Unifying aging and frailty through complex dynamical networks. Exp. Gerontol. 107, 126–129 (2018).
- 16.
Arbeev, K. G. et al. Genetics of physiological dysregulation: findings from the long life family study using joint models. Aging 12, 5920–5947 (2020).
- 17.
Lu, Y. et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020).
- 18.
Levine, M. E. Assessment of epigenetic clocks as biomarkers of aging in basic and population research. J. Gerontol. A Biol. Sci. Med. Sci. 75, 463–465 (2020).
- 19.
Li, X. et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife 9, e51507 (2020). This paper compared nine different measures of biological age, concluding that methylation age and frailty are complementary in predicting mortality.
- 20.
Kameda, M., Mikawa, T., Yokode, M., Inagaki, N. & Kondoh, H. Senescence research from historical theory to future clinical application. Geriatr. Gerontol. Int. 21, 125–130 (2021).
- 21.
Rockwood, K. & Howlett, S. E. Age-related deficit accumulation and the diseases of ageing. Mech. Ageing Dev. 180, 107–116 (2019).
- 22.
Banga, S., Heinze-Milne, S. D. & Howlett, S. E. Rodent models of frailty and their application in preclinical research. Mech. Ageing Dev. 179, 1–10 (2019).
- 23.
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O. & Rockwood, K. Frailty in elderly people. Lancet 381, 752–762 (2013).
- 24.
Andrew, M. K. & Keefe, J. M. Social vulnerability from a social ecology perspective: a cohort study of older adults from the National Population Health Survey of Canada. BMC Geriatr. 14, 90 (2014).
- 25.
Gordon, E. H. & Hubbard, R. E. Differences in frailty in older men and women. Med. J. Aust. 212, 183–188 (2020).
- 26.
O’Caoimh, R. et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 50, 96–104 (2021).
- 27.
Trendelenburg, A. U., Scheuren, A. C., Potter, P., Müller, R. & Bellantuono, I. Geroprotectors: a role in the treatment of frailty. Mech. Ageing Dev. 180, 11–20 (2019).
- 28.
Negm, A. M. et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 20, 1190–1198 (2019).
- 29.
Adja, K. Y. C. et al. The importance of taking a patient-centered, community-based approach to preventing and managing frailty: a public health perspective. Front. Public Health 8, 599170 (2020).
- 30.
Navarrete-Villanueva, D. et al. Frailty and physical fitness in elderly people: a systematic review and meta-analysis. Sports Med. 51, 143–160 (2021).
- 31.
Rezaei-Shahsavarloo, Z., Atashzadeh-Shoorideh, F., Gobbens, R. J. J., Ebadi, A. & Harouni, G. G. The impact of interventions on management of frailty in hospitalized frail older adults: a systematic review and meta-analysis. BMC Geriatr. 20, 526 (2020).
- 32.
Konopka, A. R. et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18, e12880 (2019).
- 33.
Moghtadaei, M. et al. The impacts of age and frailty on heart rate and sinoatrial node function. J. Physiol. 594, 7105–7126 (2016).
- 34.
Jansen, H. J. et al. Atrial structure, function and arrhythmogenesis in aged and frail mice. Sci. Rep. 7, 44336 (2017).
- 35.
Feridooni, H. A. et al. The impact of age and frailty on ventricular structure and function in C57BL/6J mice. J. Physiol. 595, 3721–3742 (2017).
- 36.
Kane, A. E. et al. Age, sex and overall health, measured as frailty, modify myofilament proteins in hearts from naturally aging mice. Sci. Rep. 10, 10052 (2020).
- 37.
Vaupel, J. W., Manton, K. G. & Stallard, E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454 (1979). This paper introduced the notion of variability in the rates of aging as defining frailty.
- 38.
Vaupel, J. W. et al. Biodemographic trajectories of longevity. Science 280, 855–860 (1998).
- 39.
Gavrilov, L. A. & Gavrilova, N. S. Late-life mortality is underestimated because of data errors. PLoS Biol. 17, e3000148 (2019).
- 40.
Hwang, S. W., Atia, M., Nisenbaum, R., Pare, D. E. & Joordens, S. Is looking older than one’s actual age a sign of poor health? J. Gen. Intern. Med. 26, 136–141 (2011).
- 41.
Fried, L. P. et al.; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156 (2001). This paper introduced the notion of the frailty syndrome as a phenotype.
- 42.
Mitnitski, A. B., Mogilner, A. J. & Rockwood, K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 1, 323–336 (2001). This paper introduced the concept of frailty as a state defined by the accumulation of deficits.
- 43.
Steverink, N., Slaets, J. P. J., Schuurmans, H. & Lis van, M. Measuring frailty. Development and testing of the Groningen Frailty Indicator (GFI). Gerontologist 41, 236–237 (2001).
- 44.
Dent, E., Kowal, P. & Hoogendijk, E. O. Frailty measurement in research and clinical practice: a review. Eur. J. Intern. Med. 31, 3–10 (2016).
- 45.
Gobbens, R. J., Boersma, P., Uchmanowicz, I. & Santiago, L. M. The Tilburg Frailty Indicator: new evidence for its validity. Clin. Interv. Aging 15, 265–274 (2020).
- 46.
Poh, A. W. Y. & Teo, S. P. Utility of frailty screening tools in older surgical patients. Ann. Geriatr. Med. Res. 24, 75–82 (2020).
- 47.
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. & Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008).
- 48.
Farrell, S. G., Mitnitski, A. B., Rockwood, K. & Rutenberg, A. D. Network model of human aging: frailty limits and information measures. Phys. Rev. E 94, 052409 (2016).
- 49.
Theou, O. et al. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing and Retirement in Europe. Ageing Res. Rev. 21, 78–94 (2015).
- 50.
Morley, J. E., Malmstrom, T. K. & Miller, D. K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J. Nutr. Health Aging 16, 601–608 (2012).
- 51.
Panza, F. et al. Cognitive frailty: predementia syndrome and vascular risk factors. Neurobiol. Aging 27, 933–940 (2006).
- 52.
Bunt, S., Steverink, N., Olthof, J., van der Schans, C. P. & Hobbelen, J. S. M. Social frailty in older adults: a scoping review. Eur. J. Ageing 14, 323–334 (2017).
- 53.
Kukla, M. et al. Irisin in liver cirrhosis. J. Clin. Med. 9, 3158 (2020).
- 54.
Howlett, S. E., Rockwood, M. R., Mitnitski, A. & Rockwood, K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 12, 171 (2014). This paper showed that the results of routine laboratory investigations can be combined to produce a frailty index with properties that suggest cellular and tissue deficits precede clinical features of frailty.
- 55.
Mitnitski, A. et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 13, 161 (2015).
- 56.
Rockwood, K. et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 173, 489–495 (2005).
- 57.
Johnson, R. L., McIsaac, D. I. & Mantilla, C. B. Preoperative frailty assessment: comment. Anesthesiology 133, 468–470 (2020).
- 58.
Kojima, G., Iliffe, S. & Walters, K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 47, 193–200 (2018).
- 59.
Kojima, G., Taniguchi, Y., Iliffe, S., Jivraj, S. & Walters, K. Transitions between frailty states among community-dwelling older people: a systematic review and meta-analysis. Ageing Res. Rev. 50, 81–88 (2019).
- 60.
Nguyen, Q. D. et al. Health heterogeneity in older adults: exploration in the Canadian Longitudinal Study on Aging. J. Am. Geriatr. Soc. 69, 678–687 (2021).
- 61.
Fan, J. et al. China Kadoorie Biobank Collaborative Group. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health 5, e650–e660 (2020).
- 62.
Kulminski, A. M. et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J. Am. Geriatr. Soc. 56, 898–903 (2008).
- 63.
Sathyan, S. & Verghese, J. Genetics of frailty: a longevity perspective. Transl. Res. 221, 83–96 (2020).
- 64.
Ukraintseva, S., Yashin, A. I. & Arbeev, K. G. Resilience versus robustness in aging. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1533–1534 (2016).
- 65.
Rolfson, D. B., Majumdar, S. R., Tsuyuki, R. T., Tahir, A. & Rockwood, K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 35, 526–529 (2006).
- 66.
Parks, R. J. et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J. Gerontol. A Biol. Sci. Med. Sci. 67, 217–227 (2012). This paper demonstrated that the concept of a frailty index could be applied to animal models.
- 67.
Whitehead, J. C. et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J. Gerontol. A Biol. Sci. Med. Sci. 69, 621–632 (2014).
- 68.
Liu, H., Graber, T. G., Ferguson-Stegall, L. & Thompson, L. V. Clinically relevant frailty index for mice. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1485–1491 (2014).
- 69.
Hua, J. et al. Assessment of frailty in aged dogs. Am. J. Vet. Res. 77, 1357–1365 (2016).
- 70.
Banzato, T. et al. A Frailty Index based on clinical data to quantify mortality risk in dogs. Sci. Rep. 9, 16749 (2019).
- 71.
Yamada, Y. et al. Caloric restriction and healthy life span: frail phenotype of nonhuman primates in the Wisconsin national primate research center caloric restriction study. J. Gerontol. A Biol. Sci. Med. Sci. 73, 273–278 (2018).
- 72.
McNally, M. & Lahey, W. Frailty’s place in ethics and law: some thoughts on equality and autonomy and on limits and possibilities for aging citizens. Interdiscip. Top. Gerontol. Geriatr. 41, 174–185 (2015).
- 73.
Han, L., Clegg, A., Doran, T. & Fraser, L. The impact of frailty on health care resource use: a longitudinal analysis using the Clinical Practice Research Datalink in England. Age Ageing 48, 665–671 (2019). This representative study suggests that per person cost to the health care system increases with the degree of frailty.
- 74.
Granger, K., Ninan, S. & Stopford, E. The patient presenting with ‘acopia’. Acute Med. 12, 173–177 (2013).
- 75.
Vermeiren, S. et al. Gerontopole Brussels Study group. Frailty and the prediction of negative health outcomes: a meta-analysis. J. Am. Med. Dir. Assoc. 17, 1163.e1–1163.e17 (2016).
- 76.
Shi, S. M., McCarthy, E. P., Mitchell, S. L. & Kim, D. H. Predicting mortality and adverse outcomes: comparing the frailty index to general prognostic indices. J. Gen. Intern. Med. 35, 1516–1522 (2020).
- 77.
Sillner, A. Y. et al. The association of a frailty index and incident delirium in older hospitalized patients: an observational cohort study. Clin. Interv. Aging 15, 2053–2061 (2020).
- 78.
Pérez-Zepeda, M. U., Carrillo-Vega, M. F., Theou, O., Jácome-Maldonado, L. D. & García-Peña, C. Hospital complications and frailty in Mexican older adults: an emergency care cohort analysis. Front. Med. 7, 505 (2020).
- 79.
Bowman, K. et al. Predicting incident delirium diagnoses using data from primary-care electronic health records. Age Ageing 49, 374–381 (2020).
- 80.
Hollinghurst, J. et al. External validation of the electronic Frailty Index using the population of Wales within the Secure Anonymised Information Linkage Databank. Age Ageing 48, 922–926 (2019).
- 81.
Kim, D. H. Measuring frailty in health care databases for clinical care and research. Ann. Geriatr. Med. Res. 24, 62–74 (2020).
- 82.
Simo, N. et al. Frailty index, hospital admission and number of days spent in hospital in nursing home residents: results from the INCUR study. J. Nutr. Health Aging 25, 155–159 (2021).
- 83.
Know, C. S., Hasan, S. S., Thiruchelvam, K. & Aldeyab, M. Association of frailty and mortality in patients with COVID-19: a meta-analysis. Br. J. Anaesth. 126, e108–e110 (2021).
- 84.
Pranata, R. et al. Clinical Frailty Scale and mortality in COVID-19: a systematic review and dose–response meta-analysis. Arch. Gerontol. Geriatr. 93, 104324 (2020).
- 85.
Hägg, S. et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with Coronavirus Disease 2019 in geriatric care. J. Am. Med. Dir. Assoc. 21, 1555–1559 (2020).
- 86.
Izurieta, H. S. et al. Natural history of COVID-19: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. J. Infect. Dis. 223, 945–956 (2021).
- 87.
Cosco, T. D. et al. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? a systematic review. Age Ageing 50, 608–616 (2021).
- 88.
Petermann-Rocha, F. et al. Comparison of two different frailty measurements and risk of hospitalisation or death from COVID-19: findings from UK Biobank. BMC Med. 18, 355 (2020).
- 89.
Ma, Y. et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: a prospective cohort study. BMC Med. 18, 274 (2020).
- 90.
Vilches-Moraga, A. et al. COPE Study. Increased care at discharge from COVID-19: the association between pre-admission frailty and increased care needs after hospital discharge; a multicentre European observational cohort study. BMC Med. 18, 408 (2020).
- 91.
Marengoni, A., Zucchelli, A., Grande, G., Fratiglioni, L. & Rizzuto, D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing 49, 923–926 (2020).
- 92.
Kennedy, M. et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw. Open 3, e2029540 (2020).
- 93.
Persico, I. et al. Frailty and delirium in older adults: a systematic review and meta-analysis of the literature. J. Am. Geriatr. Soc. 66, 2022–2030 (2018).
- 94.
Aucoin, S. D. et al. Accuracy and feasibility of clinically applied frailty instruments before surgery: a systematic review and meta-analysis. Anesthesiology 133, 78–95 (2020).
- 95.
Wilson, J. E. et al. Delirium. Nat. Rev. Dis. Primers 6, 90 (2020).
- 96.
Geriatric Medicine Research Collaborative. Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: results of a prospective multi-centre study on World Delirium Awareness Day. BMC Med. 17, 229 (2019).
- 97.
McElhaney, J. E. et al. The immune response to influenza in older humans: beyond immune senescence. Immun. Ageing 17, 10 (2020).
- 98.
Soiza, R. L., Scicluna, C. & Thomson, E. C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 50, 279–283 (2021).
- 99.
Andrew, M. K. & McElhaney, J. E. Age and frailty in COVID-19 vaccine development. Lancet 396, 1942–1944 (2021).
- 100.
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association Workgroup. Alzheimers Dement. 7, 263–269 (2011).
- 101.
Landau, S. M. et al. Amyloid deposition, hypometabolism and longitudinal cognitive decline. Ann. Neurol. 72, 578–586 (2012).
- 102.
Cummings, J. The National Institute on Aging-Alzheimer’s Association framework on Alzheimer’s disease: application to clinical trials. Alzheimers Dement. 15, 172–178 (2019).
- 103.
Nicoll, J. A. R. et al. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain 142, 2113–2126 (2019).
- 104.
Holmes, C. et al. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 372, 216–223 (2008).
- 105.
Lim, A. et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J. Am. Geriatr. Soc. 47, 564–569 (1999).
- 106.
Matthews, F. E. et al. Epidemiological pathology of dementia: attributable risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 6, e1000180 (2009).
- 107.
Brenowitz, W. D. et al. Alzheimer’s disease neuropathologic change, Lewy body disease and vascular brain injury in clinic- and community-based samples. Neurobiol. Aging 53, 83–92 (2017).
- 108.
Boyle, P. A. et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann. Neurol. 85, 114–124 (2019).
- 109.
Savva, G. M. et al. Age, neuropathology and dementia. N. Engl. J. Med. 360, 2302–2309 (2009).
- 110.
Wallace, L. M. K. et al. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 18, 177–184 (2019). This paper demonstrates that frailty moderates the risk of Alzheimer neuropathology in relation to Alzheimer dementia in late life.
- 111.
Mitnitski, A., Fallah, N., Rockwood, M. R. & Rockwood, K. Transitions in cognitive status in relation to frailty in older adults: a comparison of three frailty measures. J. Nutr. Health Aging 15, 863–867 (2011).
- 112.
Song, X., Mitnitski, A. & Rockwood, K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurol 77, 227–234 (2011). This paper introduced the notion of ‘traditional’ and ‘nontraditional’ risk factors for evaluating risk in relation to age-associated deficits.
- 113.
Wallace, L. M. K. et al. Neuropathological burden and the degree of frailty in relation to global cognition and dementia. Neurology 95, e3269–e3279 (2020).
- 114.
Sathyan, S. et al. Frailty and risk of incident motoric cognitive risk syndrome. J. Alzheimers Dis. 71, S85–S93 (2019).
- 115.
Livingston, G. et al. Dementia prevention, intervention and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
- 116.
Wallace, L. M. et al. Accumulation of nontraditional risk factors for coronary heart disease is associated with incident coronary heart disease hospitalization and death. PLoS ONE 9, e90475 (2014).
- 117.
Dewan, P. et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction—an analysis of PARADIGM-HF and ATMOSPHERE. Eur. J. Heart Fail. 22, 2123–2133 (2020).
- 118.
Farooqi, M. A. M., Gerstein, H., Yusuf, S. & Leong, D. P. Accumulation of deficits as a key risk factor for cardiovascular morbidity and mortality: a pooled analysis of 154,000 individuals. J. Am. Heart Assoc. 9, e014686 (2020). This large-scale independent reanalysis of several clinical trials demonstrated the inseparability of deficit-driven, variable rates of aging from cardiovascular mortality.
- 119.
Aguayo, G. A. et al. Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer and total mortality in an elderly general population in England: an observational study. PLoS Med. 15, e1002543 (2018).
- 120.
Wilkinson, C., Todd, O., Clegg, A., Gale, C. P. & Hall, M. Management of atrial fibrillation for older people with frailty: a systematic review and meta-analysis. Age Ageing 48, 196–203 (2019).
- 121.
Clegg, A. et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 45, 353–360 (2016). This paper introduced an electronic frailty index collected from routine primary care practice visits, offering proof-of-concept of routine screening of the degree of frailty.
- 122.
Wilkinson, C. et al. Atrial fibrillation and oral anticoagulation in older people with frailty: a nationwide primary care electronic health records cohort study. Age Ageing 50, 772–779 (2021).
- 123.
Wilkinson, C. et al. Clinical outcomes in patients with atrial fibrillation and frailty: insights from the ENGAGE AF-TIMI 48 trial. BMC Med. 18, 401 (2020).
- 124.
Warwick, J. et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 13, 78 (2015).
- 125.
Pajewski, N. M. et al.; SPRINT Study Research Group. Characterizing frailty status in the systolic blood pressure intervention trial. J. Gerontol. A Biol. Sci. Med. Sci. 71, 649–655 (2016).
- 126.
Russo, G. et al. Impact of SPRINT results on hypertension guidelines: implications for ‘frail’ elderly patients. J. Hum. Hypertens. 32, 633–638 (2018).
- 127.
Benetos, A., Petrovic, M. & Strandberg, T. Hypertension management in older and frail older patients. Circ. Res. 124, 1045–1060 (2019).
- 128.
Giffin, A., Madden, K. M. & Hogan, D. B. Blood pressure targets for older patients—do advanced age and frailty really not matter? Can. Geriatr. J. 23, 205–209 (2020).
- 129.
Bartosch, P., McGuigan, F. E. & Akesson, K. E. Progression of frailty and prevalence of osteoporosis in a community cohort of older women-a 10-year longitudinal study. Osteoporos. Int. 29, 2191–2199 (2018).
- 130.
Guaraldi, G. et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 29, 1633–1641 (2015).
- 131.
Legge, A. et al. Prediction of damage accrual in systemic lupus erythematosus using the systemic lupus international collaborating clinics frailty index. Arthritis Rheumatol. 72, 658–666 (2020).
- 132.
Herrera, A. P. et al. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am. J. Public Health 100, S105–S112 (2010).
- 133.
Rich, M. W. et al.; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology and American Geriatrics Society. J. Am. Coll. Cardiol. 67, 2419–2440 (2016).
- 134.
Walker, D. M. et al. Frailty and the management of patients with acute cardiovascular disease: a position paper from the Acute Cardiovascular Care Association. Eur. Heart J. Acute Cardiovasc. Care. 7, 176–193 (2018).
- 135.
Hanlon, P. et al. Identifying frailty in trials: an analysis of individual participant data from trials of novel pharmacological interventions. BMC Med. 18, 309 (2020).
- 136.
Motta, F., Sica, A. & Selmi, C. Frailty in rheumatic diseases. Front. Immunol. 11, 576134 (2020).
- 137.
Andrew, M. K., Mitnitski, A., Kirkland, S. A. & Rockwood, K. The impact of social vulnerability on the survival of the fittest older adults. Age Ageing 41, 161–165 (2012). This paper proposes that the outcomes of the fittest people define the characteristics of a population or group, demonstrated here by the impact of social vulnerability on health and mortality.
- 138.
Theou, O. et al. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing 42, 614–619 (2013).
- 139.
Shamliyan, T., Talley, K. M., Ramakrishnan, R. & Kane, R. L. Association of frailty with survival: a systematic literature review. Ageing Res. Rev. 12, 719–736 (2013).
- 140.
Ellis, H. L. et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ 192, E3–E8 (2020).
- 141.
Abeliansky, A. L. & Strulik, H. Hungry children age faster. Econ. Hum. Biol. 29, 211–220 (2018).
- 142.
Marshall, A., Nazroo, J., Tampubolon, G. & Vanhoutte, B. Cohort differences in the levels and trajectories of frailty among older people in England. J. Epidemiol. Community Health 69, 316–321 (2015).
- 143.
Bäckman, K. et al. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg, Sweden. J. Gerontol. A Biol. Sci. Med. Sci. 72, 945–950 (2017).
- 144.
Mousa, A. et al. Is frailty a stable predictor of mortality across time? evidence from the cognitive function and ageing studies. Age Ageing 47, 721–727 (2018).
- 145.
Yu, R. et al. Trajectories of frailty among Chinese older people in Hong Kong between 2001 and 2012: an age–period–cohort analysis. Age Ageing 47, 254–261 (2018).
- 146.
Hoogendijk, E.O. et al. Trends in frailty and its association with mortality: results from the Longitudinal Aging Study Amsterdam, 1995–2016. Am. J. Epidemiol. https://doi.org/10.1093/aje/kwab018 (2021).
- 147.
Haapanen, M. J. et al. Infant and childhood growth and frailty in old age: the Helsinki Birth Cohort Study. Aging Clin. Exp. Res. 31, 717–721 (2019).
- 148.
Welstead, M. et al. Inflammation as a risk factor for the development of frailty in the Lothian Birth Cohort 1936. Exp. Gerontol. 139, 111055 (2020).
- 149.
Rockwood, K., Song, X. & Mitnitski, A. Changes in relative fitness and frailty across the adult life span: evidence from the Canadian National Population Health Survey. CMAJ 183, E487–E494 (2011).
- 150.
Blodgett, J. M., Rockwood, K. & Theou, O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev. 2, E96–E104 (2021).
- 151.
Brothers, T. D., Theou, O. & Rockwood, K. Frailty and migration in middle-aged and older Europeans. Arch. Gerontol. Geriatr. 58, 63–68 (2014).
- 152.
Franse, C. B. et al. Ethnic differences in frailty: a cross-sectional study of pooled data from community-dwelling older persons in the Netherlands. BMJ Open. 8, e022241 (2018).
- 153.
Pradhananga, S. et al. Ethnic differences in the prevalence of frailty in the United Kingdom assessed using the electronic Frailty Index. Aging Med. 2, 168–173 (2019).
- 154.
Herr, M., Robine, J. M., Aegerter, P., Arvieu, J. J. & Ankri, J. Contribution of socioeconomic position over life to frailty differences in old age: comparison of life-course models in a French sample of 2,350 old people. Ann. Epidemiol. 25, 674–680 (2015).
- 155.
Van der Linden, B. W. A. et al. Life-course socioeconomic conditions and frailty at older ages. J. Gerontol. B Psychol. Sci. Soc. Sci. 75, 1348–1357 (2020).
- 156.
Young, A. C., Glaser, K., Spector, T. D. & Steves, C. J. The identification of hereditary and environmental determinants of frailty in a cohort of UK twins. Twin Res. Hum. Genet. 19, 600–609 (2016). This twin study suggests that about half of the interindividual variation in frailty is heritable.
- 157.
Li, X. et al. The frailty index is a predictor of cause-specific mortality independent of familial effects from midlife onwards: a large cohort study. BMC Med. 17, 94 (2019).
- 158.
Raymond, E. et al. Drivers of frailty from adulthood into old age: results from a 27-year longitudinal population-based study in Sweden. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1943–1950 (2020).
- 159.
Sathyan, S. et al. Plasma proteomic profile of age, health span and all-cause mortality in older adults. Aging Cell 19, e13250 (2020).
- 160.
Livshits, G. et al. Multi-omics analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain 159, 2565–2572 (2018).
- 161.
Taneja, S., Mitnitski, A. B., Rockwood, K. & Rutenberg, A. D. Dynamical network model for age-related health deficits and mortality. Phys. Rev. E 93, 022309 (2016). The paper introduced a framework for modeling deficit accumulation and its relationship to mortality.
- 162.
Jazwinski, S. M. & Kim, S. Examination of the dimensions of biological age. Front. Genet. 10, 263 (2019).
- 163.
Araújo Carvalho, A. C. et al. Telomere length and frailty in older adults—a systematic review and meta-analysis. Ageing Res. Rev. 54, 100914 (2019).
- 164.
Hägg, S., Jylhävä, J., Wang, Y., Czene, K. & Grassmann, F. Deciphering the genetic and epidemiological landscape of mitochondrial DNA abundance. Hum. Genet. 40, 849–861 (2021). This paper demonstrates that mitochondrial dysfunction is related to the degree of frailty in a sex-specific fashion.
- 165.
Hao, Q. et al. Prediction of mortality in Chinese very old people through the frailty index based on routine laboratory data. Sci. Rep. 9, 221 (2019).
- 166.
King, K. E., Fillenbaum, G. G. & Cohen, H. J. A cumulative deficit laboratory test-based frailty index: personal and neighborhood associations. J. Am. Geriatr. Soc. 65, 1981–1987 (2017).
- 167.
Mitnitski, A. & Rockwood, K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology 17, 199–204 (2016).
- 168.
Hoogendijk, E. O. et al. Tracking changes in frailty throughout later life: results from a 17-year longitudinal study in the Netherlands. Age Ageing 47, 727–733 (2018).
- 169.
Stolz, E., Hoogendijk, E.O., Mayerl, H. & Freidl, W. Frailty changes predict mortality in four longitudinal studies of aging. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glaa266 (2020).
- 170.
Stolz, E. et al. Acceleration of health deficit accumulation in late life: evidence of terminal decline in frailty index three years before death in the US Health and Retirement Study. Ann. Epidemiol. 58, 156–161 (2021).
- 171.
Lu, W. et al. Relationship between employment histories and frailty trajectories in later life: evidence from the English Longitudinal Study of Ageing. J. Epidemiol. Community Health 71, 439–445 (2017).
- 172.
Noppert, G. A., Aiello, A. E., O’Rand, A. M. & Cohen, H. J. Race/ethnic and educational disparities in the association between pathogen burden and a laboratory-based cumulative deficits index. J. Racial Ethn. Health Disparities 7, 99–108 (2020).
- 173.
Miller, M. G., Thangthaeng, N. & Shukitt-Hale, B. A clinically relevant frailty index for aging rats. J. Gerontol. A Biol. Sci. Med. Sci. 72, 892–896 (2017).
- 174.
Rockwood, K. et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci. Rep. 7, 43068 (2017). This paper demonstrates that deficits accumulate in similar patterns across the life course in humans and in mice.
- 175.
Baumann, C. W., Kwak, D. & Thompson, L. V. Assessing onset, prevalence and survival in mice using a frailty phenotype. Aging 10, 4042–4053 (2018).
- 176.
Kwak, D., Baumann, C. W. & Thompson, L. V. Identifying characteristics of frailty in female mice using a phenotype assessment tool. J. Gerontol. A Biol. Sci. Med. Sci. 75, 640–646 (2020).
- 177.
Yorke, A., Kane, A. E., Hancock Friesen, C. L., Howlett, S. E. & O’Blenes, S. Development of a rat clinical frailty index. J. Gerontol. A Biol. Sci. Med. Sci. 72, 897–903 (2017).
- 178.
Pandey, A., Kitzman, D. & Reeves, G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment and management. JACC Heart Fail. 7, 1001–1011 (2019).
- 179.
Bibas, L. et al. Implications of frailty in elderly patients with electrophysiological conditions. JACC Clin. Electrophysiol. 2, 288–294 (2016).
- 180.
Yabuuchi, J. et al. Association of advanced glycation end products with sarcopenia and frailty in chronic kidney disease. Sci. Rep. 10, 17647 (2020).
- 181.
Antoch, M. P. et al. Physiological frailty index: quantitative in-life estimate of individual biological age in mice. Aging 9, 615–626 (2017).
- 182.
Keller, K., Kane, A. E., Heinze-Milne, S., Grandy, S. A. & Howlett, S. E. Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro- and anti-inflammatory cytokines in aging male and female C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1149–1157 (2019). This paper shows that long-term treatment of older mice with a drug that reduces inflammation improves health span.
- 183.
Kane, A. E., Keller, K. M., Heinze-Milne, S., Grandy, S. A. & Howlett, S. E. A murine frailty index based on clinical and laboratory measurements: links between frailty and pro-inflammatory cytokines differ in a sex-specific manner. J. Gerontol. A Biol. Sci. Med. Sci. 74, 275–282 (2019).
- 184.
Wilson, D. et al. Frailty is associated with neutrophil dysfunction which is correctable with phosphoinositol-3-kinase inhibitors. J. Gerontol. A Biol. Sci. Med. Sci. 75, 2320–2325 (2020).
- 185.
Fulop, T. et al. Frailty, inflammation and immunosenescence. Interdiscip. Top. Gerontol. Geriatr. 41, 26–40 (2015).
- 186.
Graber, T. G., Ferguson-Stegall, L., Liu, H. & Thompson, L. V. Voluntary aerobic exercise reverses frailty in old mice. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1045–1058 (2015).
- 187.
Gomez-Cabrera, M. C. et al. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J. Gerontol. A Biol. Sci. Med. Sci. 72, 885–891 (2017).
- 188.
Seldeen, K. L. et al. High-intensity interval training improves physical performance and frailty in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 73, 429–437 (2018).
- 189.
Seldeen, K. L. et al. High-intensity interval training improves physical performance in aged female mice: a comparison of mouse frailty assessment tools. Mech. Ageing Dev. 180, 49–62 (2019).
- 190.
Kane, A. E. et al. Impact of longevity interventions on a validated mouse clinical frailty index. J. Gerontol. A Biol. Sci. Med. Sci. 71, 333–339 (2016).
- 191.
Correia-Melo, C. et al. Rapamycin improves health span but not inflammaging in Nfκb1−/− mice. Aging Cell. 18, e12882 (2019).
- 192.
Yu, D. et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for life span extension. Cell Rep. 29, 236–248 (2019).
- 193.
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 (2017).
- 194.
Richardson, N. E. et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging 1, 73–86 (2021).
- 195.
Henderson, Y.O. et al. Late-life intermittent fasting decreases aging-related frailty and increases renal hydrogen sulfide production in a sexually dimorphic manner. Geroscience https://doi.org/10.1007/s11357-021-00330-4 (2021).
- 196.
Guderyon, M. J. et al. Mobilization-based transplantation of young-donor hematopoietic stem cells extends life span in mice. Aging Cell 19, e13110 (2020).
- 197.
Schultz, M. B. et al. Age and life expectancy clocks based on machine-learning analysis of mouse frailty. Nat. Commun. 11, 4618 (2020).
- 198.
Liu, Y. et al. Allicin reversed the process of frailty in aging male Fischer 344 rats with osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 75, 821–825 (2020).
- 199.
Asadi Shahmirzadi, A. et al. Alpha-ketoglutarate, an endogenous metabolite, extends life span and compresses morbidity in aging mice. Cell Metab. 32, 447–456 (2020).
- 200.
Chellappa, K. et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell. 18, e13014 (2019).
- 201.
Mach, J. et al. Chronic polypharmacy with increasing Drug Burden Index exacerbates frailty and impairs physical function, with effects attenuated by de-prescribing, in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1010–1018 (2020).
- 202.
Fielder, E. et al. Sublethal whole-body irradiation causes progressive premature frailty in mice. Mech. Ageing Dev. 180, 63–69 (2019).
- 203.
Darvall, J. N. et al. Frailty and outcomes from pneumonia in critical illness: a population-based cohort study. Br. J. Anaesth. 125, 730–738 (2020).
- 204.
Pulok, M. H., Theou, O., van der Valk, A. M. & Rockwood, K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing 49, 1071–1079 (2020).
- 205.
Rockwood, K. & Mitnitski, A. Limits to deficit accumulation in elderly people. Mech. Ageing Dev. 127, 494–496 (2006).
- 206.
McIsaac, D. I., MacDonald, D. B. & Aucoin, S. D. Frailty for perioperative clinicians: a narrative review. Anesth. Analg. 130, 1450–1460 (2020).
- 207.
Carli, F., Bessissow, A., Awasthi, R. & Liberman, S. Prehabilitation: finally utilizing frailty screening data. Eur. J. Surg. Oncol. 46, 321–325 (2020).
- 208.
Coelho-Júnior, H. J. et al. Evidence-based recommendations for resistance and power training to prevent frailty in community-dwellers. Aging Clin. Exp. Res. 33, 2069–2086 (2021).
- 209.
Guralnik, J. et al. Clinically meaningful change for physical performance: perspectives of the ICFSR Task Force. J. Frailty Aging 9, 9–13 (2020).
- 210.
Jang, I. Y. et al. Evaluation of clinically meaningful changes in measures of frailty. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1143–1147 (2020).
- 211.
Theou, O. et al. Exploring clinically meaningful changes for the frailty index in a longitudinal cohort of hospitalized older patients. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1928–1934 (2020).
- 212.
Hshieh, T. T., Yang, T., Gartaganis, S. L., Yue, J. & Inouye, S. K. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am. J. Geriatr. Psychiatry 26, 1015–1033 (2018).
- 213.
Curtis, M. S., Forman, N. A., Donovan, A. L. & Whitlock, E. L. Postoperative delirium: why, what and how to confront it at your institution. Curr. Opin. Anaesthesiol. 33, 668–673 (2020).
- 214.
Khachaturian, A. S. et al. International drive to illuminate delirium: a developing public health blueprint for action. Alzheimers Dement. 16, 711–725 (2020).
- 215.
Rockwood, K. et al. CCCDTD5: reducing the risk of later-life dementia. Evidence informing the Fifth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD-5). Alzheimers Dement. 6, e12083 (2020).
- 216.
Kirkland, J. L. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518–536 (2020).
- 217.
Williams, D. M., Jylhävä, J., Pedersen, N. L., N.L. & Hägg, S. A frailty index for UK Biobank participants.J. Gerontol. A Biol. Sci. Med. Sci. 74, 582–587 (2019).
- 218.
Wang, Q. et al. Genetically predicted life-long lowering of low-density lipoprotein cholesterol is associated with decreased frailty: a Mendelian randomization study in UK biobank. EBioMedicine 45, 487–494 (2019).
- 219.
Mekli, K. Frailty Index associates with GRIN2B in two representative samples from the United States and the United Kingdom. PLoS ONE 13, e0207824 (2018).
- 220.
Espeland, M. A. et al.; Multimorbidity Clinical Trials Consortium. Clinical trials targeting aging and age-related multimorbidity.72, 355–361 (2017). J. Gerontol. A Biol. Sci. Med. Sci. 72, 355–361 (2017).
- 221.
Warner, H. R. et al. Program for testing biological interventions to promote healthy aging. Mech. Ageing Dev. 115, 199–207 (2000).
- 222.
Nadon, N. L. et al. Design of aging intervention studies: the NIA interventions testing program. Age 30, 187–199 (2008).
- 223.
Farrell, S., Mitnitski, A., Rockwood, K. & Rutenberg, A. Generating synthetic aging trajectories with a weighted network model using cross-sectional data. Sci. Rep. 10, 19833 (2020).
- 224.
García-Peña, C. et al. Network analysis of frailty and aging: empirical data from the Mexican Health and Aging Study. Exp. Gerontol. 128, 110747 (2019).
- 225.
Stubbings, G., Farrell, S., Mitnitski, A., Rockwood, K. & Rutenberg, A. Informative frailty indices from binarized biomarkers. Biogerontology 70, 1–11 (2020).
- 226.
Farrell, S., Stubbings, G., Rockwood, K., Mitnitski, A. & Rutenberg, A. The potential for complex computational models of aging. Mech. Ageing Dev. 193, 111403 (2021).
Acknowledgements
We wish to acknowledge the influence of Arnold Mitnitski to how we think about frailty. Arnold died after a brief illness on 26 May 2021. We will miss him both as a friend and colleague, and one who has had an enormous influence on our field. Work in the laboratory of S.E.H. is supported the Canadian Institutes of Health Research (PJT 162462 and 155961) and the Heart and Stroke Foundation of Canada (G-19–0026260). Work in the laboratory of A.D.R. is supported by grants from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2019–05888). Work in the Geriatric Medicine Research Unit (GMRU; K.R.) is supported by grants from the Canadian Institutes of Health Research (PJT 156114), Research Nova Scotia (RNS-SIG-2021–1640) and the Canadian Frailty Network (CFN-CSA-2019 and NSHA-2020). The GMRU has received long-term philanthropic support from the Fountain Family Innovation Fund of the QEII Health Sciences Foundation. The figures were created with BioRender.com.
Author information
Affiliations
Contributions
S.E.H. and K.R. conceived the review and wrote the first version of the manuscript. A.D.R. provided constructive input and wrote additional sections in subsequent revisions of the manuscript. All authors approved the final version of the article and figures.
Corresponding author
Ethics declarations
Competing interests
K.R. has asserted copyright of the Clinical Frailty Scale through Dalhousie University’s Industry, Liaison and Innovation Office. Use is free for education, research and not-for-profit health care. Users agree not to change or commercialize the scale. In addition to academic and hospital appointments, K.R. is cofounder of Ardea Outcomes, which (as DGI Clinical) in the last 3 years has contracts with pharma and device manufacturers (Biogen, Hollister, Novartis, Nutricia, Roche and Takeda) on individualized outcome measurement. In 2019, K.R. was paid an honorarium for an interview with Biogen. In 2020, he attended an advisory board meeting with Nutricia on dementia, and chaired a scientific workshop and technical review panel on frailty for the Singapore National Research Foundation. Otherwise, any personal fees were for invited guest lectures, rounds and academic symposia, received directly from event organizers, for presentations on frailty. K.R. is associate director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes for Health Research, the Alzheimer Society of Canada and several other charities. S.E.H. has a paid consulting role with Ardea Outcomes. A.D.R. has no competing interests to declare.
Additional information
Peer review information Nature Aging thanks Simon Conroy, Luigi Ferrucci, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Howlett, S.E., Rutenberg, A.D. & Rockwood, K. The degree of frailty as a translational measure of health in aging. Nat Aging 1, 651–665 (2021). https://ift.tt/3xId80G
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
"degree" - Google News
August 12, 2021 at 10:01PM
https://ift.tt/3AC4pPh
The degree of frailty as a translational measure of health in aging - Nature.com
"degree" - Google News
https://ift.tt/2zPqEHn
https://ift.tt/2WkjZfX
Bagikan Berita Ini
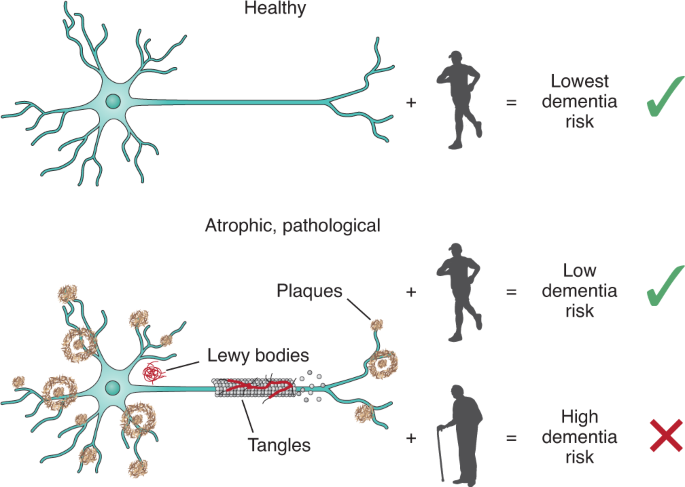
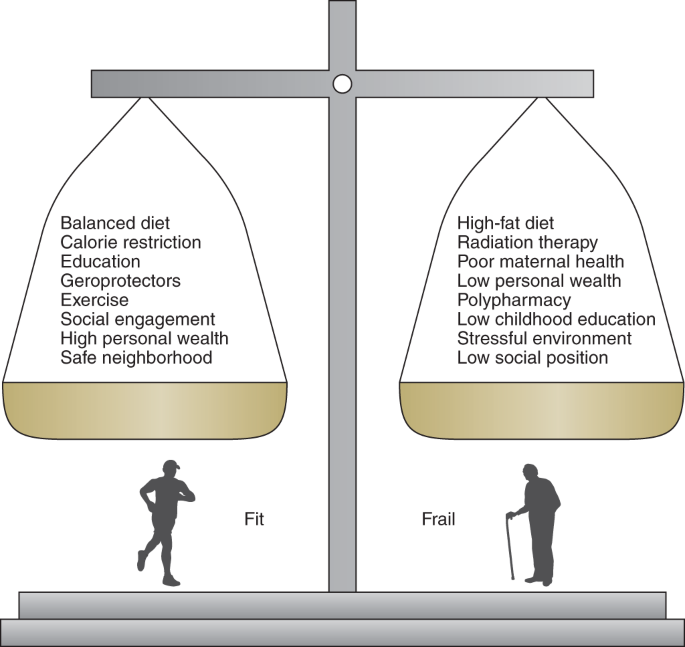
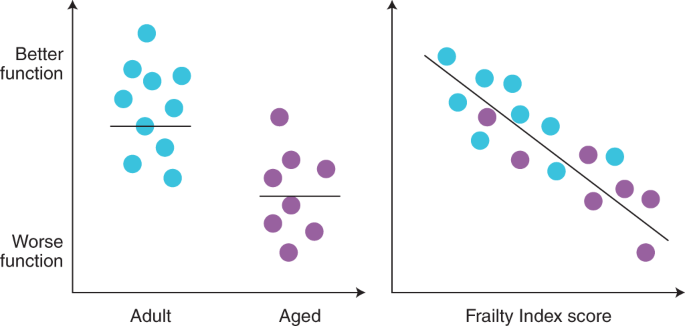
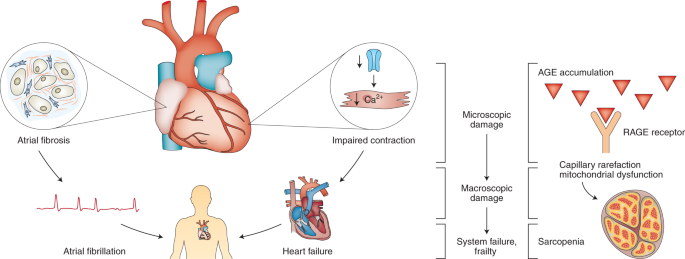















0 Response to "The degree of frailty as a translational measure of health in aging - Nature.com"
Post a Comment